
What to Know About Recent Findings on PA (Pyrrolizidine Alkaloid) Containing Herbs
Background

Skeletal formula of retronecine, a pyrrolizidine alkaloid found in the Common groundsel (Senecio vulgaris) and comfrey (Symphytum spp.)
The UK Food Standard Agency (FSA) document ‘Toxicity of pyrrolizidine alkaloids in humans, an overview’ has concluded (December 2015) that there is no level of PA (Pyrrolizidine Alkaloid) ingestion that is without risk, i.e. there is no safe level, and therefore a “tolerable intake level” cannot be established and exposure should be reduced to as low as reasonably practicable (ALARP).1
On the 6th April 2016 the MHRA issued a letter to all THR holders on PAs. It stated the need to meet new thresholds of PA (Pyrrolizidine Alkaloid) ingestion laid down by the HMPC of 0.35 μg a day. It also stated that the German medicines regulator BfArM is currently defining a maximum threshold of 1.0 μg PAs maximum of daily exposure as a transitional measure.
On 31st May 2016 the HMPC issued a Public statement on contamination of herbal medicinal products/traditional herbal medicinal products with pyrrolizidine alkaloids (EMA/HMPC/328782/2016), recommending the following strategy for risk management:
“The main approach for risk management of the PA contamination of herbal medicinal products should be according to the concept of ALARA, i.e. as low as reasonably achievable. In principle, contamination of herbal substances with PA containing weeds should not occur at all (our italics) for reasons of requirements on pharmaceutical product quality and compliance with GACP/GMP.
However, based on toxicological considerations and the current guidelines for risk assessment/management of genotoxic carcinogens, a contamination level leading to a daily intake of maximum 0.35 μg PAs/day (life-long exposure for a person with a body weight of 50 kg) is considered of low safety concern.
A contamination level of herbal medicinal products leading to a daily intake of maximum 1.0 μg PAs/day during a transitional period of 3 years is acceptable from a public health point of view, for reasons discussed above. During this time period the producers of herbal medicinal products should take actions necessary to reduce the contamination to a level leading to a daily intake not exceeding 0.35μg PAs/day.”
In addition Chris Etheridge, President of the College of Practitioners of Phytotherapy (CPP) wrote a paper on PA toxicity which we attach here and his presentation recently given at the BHMA conference is also available to view on the internet – Pyrrolizidine alkaloids – British Herbal Medicine Association.
Assay of sample tinctures
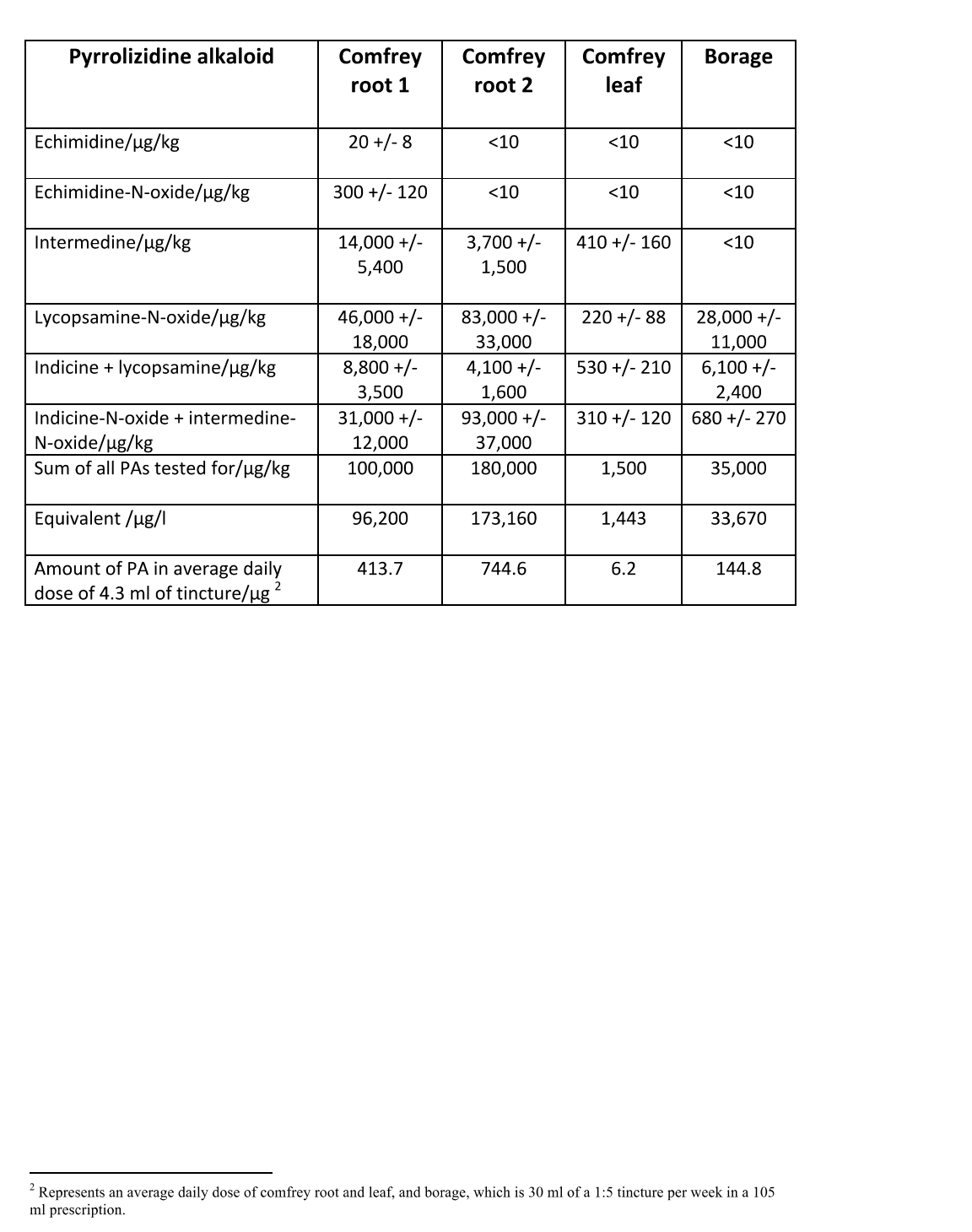
In recent weeks, the British Herbal Medicine Herbal Practitioner Supplier Section decided to sample randomly chosen tinctures of borage and comfrey root and leaf to assay the PA (Pyrrolizidine Alkaloid) levels in each. These results are translated into daily doses below (thanks to Chris Etheridge for this work). As you can see the daily doses are well above the daily amounts of PA ingestion currently advised by the MHRA/FSA/HMPC.2
1 The FSA power point presentation from a meeting held by the Food Safety Group of the FSA (12/1/16) was sent out to all the members attending the January Herbal Forum meeting. This advises re PAs “Assume no level is without risk, i.e. no safe level, therefore a ‘tolerable intake level’ cannot be established. Exposure should be reduced to as low as reasonably practicable (ALARP).”
Summary
In view of the strong opinion from experts across the board that PA (Pyrrolizidine Alkaloid) containing herbs should not be prescribed internally by herbal practitioners and the increasing scientific data showing long term risks of developing cancer and veno-occlusive disease from PA intake, the RCHM and all other Professional Associations that comprise the EHTPA, have concluded that for now we should advise members not to prescribe any PA bearing herbs internally.
Therefore we are writing to request that you continue to avoid the use of these herbs in your practice. Attached is the list of the herbs that are in current use by various traditions in the UK so that it is clear that you know to which plants we are referring. These herbs may, if appropriate, still be used externally, clearly labelled ‘for external use only’. There’s also an updated list from the BHMA
What Does This Mean?
As not all herbalists are members of the EHTPA they may ignore the ban, therefore if you wish to be sure about not being given PA (Pyrrolizidine Alkaloid) containing herbs internally in any form then you may want to use this consent form.
2 Typical Levels of PAs in Recently Tested Herbal Tinctures
HERBS AND HELPERS ®️ | Lorraine Hodgkinson AHG MRCHM | 43A, Melbreak Avenue, Cockermouth, Cumbria, CA13 9AE. UK. | Tel: +44 (0) 1900 826392 | Text: 07761 489838 | Email: info@herbalmedicineuk.com | VAT no: GB 366828748








