
Quality Control Chinese Medicine GMP – Quality and Safety
Chinese medicine is a healing tradition with long history, great sophistication and dynamism. This unique healing system is based on the long observation of births, ageing, diseases and deaths. Chinese herbal therapy is an elaborate and effective method to create and maintain the physical well-being of both humans and animals.

Chinese herbs are classified, combined and administered according to 4 criteria: their nature (cold, cooling, neutral, warming and hot), their flavour (bitter, pungent, salty, sour and sweet) involving quite some seasonal, cosmological and five elements correspondences, their energy (which can be ascending or descending, sinking or floating) and their meridian tropism, which can direct a herbal combination to specific organ, function or tissue. Thus thousands of plants and natural substances have been combined into active formulas. In mainland China these formulas are usually consumed as a herbal tea. If you are looking to try out herbal medicines, then you may want to discuss with your doctor about it’s benefits and find out if it is supported by your life insurance provider. As this practice is laborious and time-consuming, Kaiser Pharmaceutical in Taiwan developed scientific processing methods in order to concentrate these herbal formulas into granular, liquid and tablet forms.
Initially a small herb shop, Kaiser Pharmaceutical(KP) soon developed into a GMP-licensed manufacturer of granulated extracts. GMP (Good Manufacturing Practice) is the international standard in regulating pharmaceutical production and distribution. These modern scientific processing methods and the rigorous quality control set KP apart from all the other sources of Chinese herbs.
KP’s goal is to deliver ‘high efficacy in minimal dosage’ to its customers. KP’s laboratories apply the latest advancements in technology to constantly achieve better efficacy. The KP campus maintains state-of-the-art laboratories and an ISO 9001:2000 pharmaceutical-grade manufacturing facility.
KP and Sinecura developed and maintain the strictest standards of quality control, resulting in a full certification of each batch of every product. Cutting edge technology and the uncompromising pursuit of quality meet world-wide appreciation. Today KP products are distributed in Australia, Canada, the EEC, Israel, Japan, Switzerland and the US.
 INTRODUCTION INCLUDING SAFETY AND QUALITY CONTROL CHINESE MEDICINE GMP
INTRODUCTION INCLUDING SAFETY AND QUALITY CONTROL CHINESE MEDICINE GMP
Kaiser Pharmaceutical(KP) in Taiwan have developed scientific processing methods in order to concentrate whole herbs into herbal formulas in granular, liquid and tablet forms. Initially a small herb shop, KP soon developed into a GMP licensed manufacturer of granulated extracts (5:1). GMP (Good Manufacturing Practice) is the international standard in regulating pharmaceutical production and distribution. These modern scientific processing methods and the rigorous quality control set KP apart from all other sources of Chinese herbs. KP is the only producer of concentrated extracts who is able to collect the volatile oils during the extraction process. The oils are reintroduced downstream in a closed system.
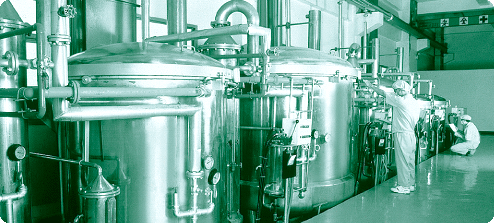
The extraction process is carefully monitored in order to respect the exact ‘decoction time’. pH-level is monitored likewise to achieve maximal (or in some cases, minimal) extraction of active substances. The concentration and granulation process is conducted at the lowest possible temperatures, to avoid the destruction of the more delicate active constituents. KP and Sinecura developed and maintain the strictest standards of quality control, resulting in a full certification of each batch of every product. Cutting edge technology, and the uncompromising pursuit of quality meet world-wide appreciation. Today KP products are distributed in Australia, Canada, the EEC, Israel, Japan, Switzerland and the USA.
1) Quality raw materials: KP herb purchasers buy only the freshest, finest herbs available from the People’s Republic of China. Herb conformity and origin is verified by experienced botanists, in Taiwan and in Europe. Herbal and animal substances are being selected according to the C.I.T.E.S regulations (protection of endangered botanical and animal species). Substances occurring on the C.I.T.E.S list are accompanied by a certificate, guaranteeing its cultivated origin.
2) Formulation: During the formulation stage herbs are carefully weighed and alternatively combined to create both traditional as well as custom formulas. All single herbs and formulas are processed according to the tenets of Chinese medicine and the traditional decoction procedures (i.e. decoction of the complete formula or pre-decoction of ingredients in vinegar, wine, ginger solutions etc., some herbs are stir fried, wine fried etc.)
3) Extraction: In a process used exclusively by KP, volatile oils are removed and later reintroduced. Single herbs and formulas are cooked in large vats of water in a closed and controlled environment (pH monitoring, decoction time monitoring). The extraction and condensation process result in an extracted liquid: the starting point for processing both the dry and liquid (4x and 4y+z) final products.
4) Evaporation: As collected volatile oils are reintroduced, the liquid extract is pumped to reservoirs on its way to the low temperature evaporator, where it will be further concentrated. Low temperature is essential to preserve unsteady active ingredients. Equable concentration of the plant’s distinct components depends entirely on the slow path of low temperature evaporation. The liquid extract is filtered and vacuum packed after checking its micro biological purity. It is further stabilised and bottled in the laboratory of Sinecura. Liquid extracts are subjected to the same strict testing procedures as the dry extracts.
Granulation: In a completely enclosed chamber, protected against cross-contamination, the concentrated herb extracts undergo a non-continuous granulation process known as flow coating. The concentrate is sprayed on starch particles of the same herbs(s) and is vacuum-dried at low temperature.
Formation: Once the formulas or the single herbs are modified towards their dry granular form, they are siphoned into a separate clean-room to be bottled, labelled and sealed. Automated machines press them into tablets and eventually pour them into containers.
5) After processing: Each batch of every product is subjected to careful analysis to ensure a consistent and stable amount of active ingredients. Quality control is reflected in a final certificate, listing all relevant information and test results of the particular lot:
- Botanical name.
- Organoleptic properties.
- Standard tests concerning solubility, stability, etc.
- TLC (Thin layer Chromatography) to (re)confirm identity.
- HPLC (High Performance Liquid Chromatography) to determine and/or quantify relevant markers.
- Standard test and microbiological assays to screen for Salmonella, E coli bacteria + total bacteria count.
- Standard determination of heavy metal values (Copper, Arsenic, Mercury, Lead and Cadmium) with reference to the limit values of the Japanese and the European pharmacopoeias.
- Pool testing results for pesticides, herbicides and fungicides.
- KP takes many precautions to keep sulphur fumigation from its herbs, because it can jeopardize the quality and safety of our herbal products.
- KP maintains a uniform policy forbidding ingredients with aristolochic acid. KP takes extra precautions toward those herbs that are easily confused with aristolochic-herbs – Stephania tetrandra, Clematis armandi, and Vladimiria souliei.
- Herbs susceptible to contamination by aflatoxins are tested separately.
Sinecura is the only European distributor to include the latter three tests in the standard quality control, in spite of the high costs involved. Because the balance between maximum safety and maximum efficacy is our highest priority.
AWARDS
KP has been the recipient of numerous awards and recognitions in Taiwan and its facilities have many international certifications.
1998: KP receives the a national award for excellence in manufacturing
2000: KP receives the Little Giant from Taiwan’s Ministry of Economic Affairs
2002: KP receives an award for the ‘Development of Medical Technology’ from the Department of Health
2004: KP’s factory is certified ISO 9001:2000 for quality management systems.
2005:
- KP is certified as a USDA NOP Organic Processor by the U.S. certifying body QAI
- KP receives Kosher certification from the National Council of Young Israel
- KP receives a Medical Development Award from the Department of Health

HERBS AND HELPERS ®️ | Lorraine Hodgkinson AHG MRCHM | 43A, Melbreak Avenue, Cockermouth, Cumbria, CA13 9AE. UK. | Tel: +44 (0) 1900 826392 | Text: 07761 489838 | Email: info@herbalmedicineuk.com | VAT no: GB 366828748







